Determination of Cd (ppb) in Water Using Stripping Voltammetry
|
|
Time to read 5 min

|
|
Time to read 5 min
Differential Pulse Stripping Voltammetry (DPASV) is a powerful electrochemical analytical technique that allows for the detection and quantification of a variety of metal ion species at very low concentrations in aqueous media. This technique consists of two main steps; Accumulation (preconcentration) and Stripping. The first step (Accumulation) is the electroplating of the analyte of interest on the electrode surface at a constant potential, followed by the second step (Stripping) which involves a potential sweep during which the preconcentrated analyte is stripped back to the solution, a process resulting in a current response proportional to the analyte concentration in the sample.
The advantages of DPASV include sensitivity (extremely low detection in ppb), high accuracy, speed and modest cost.
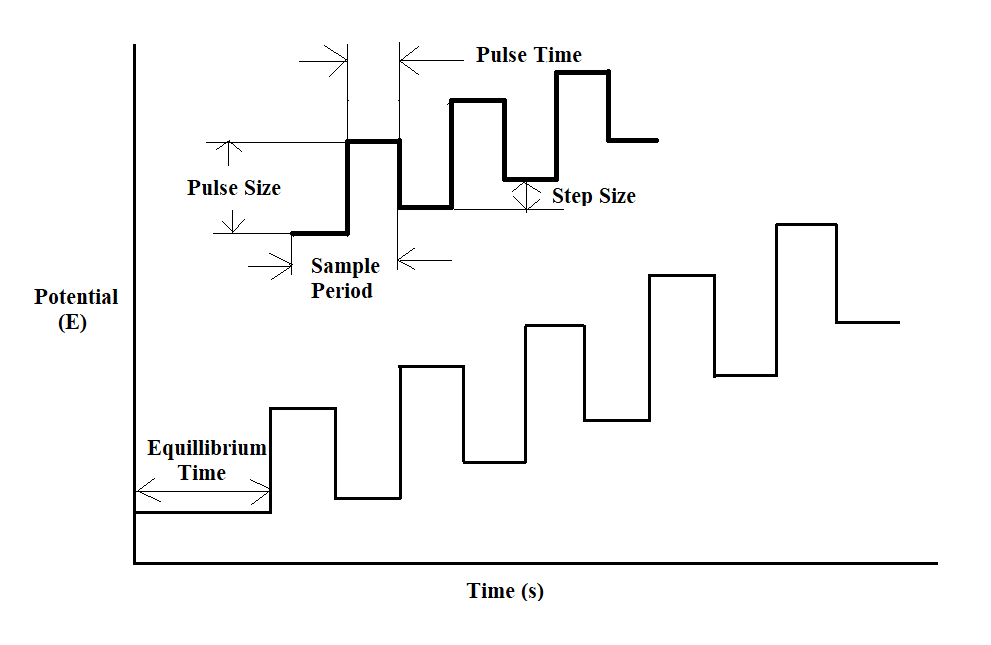
Figure 1: Potential wave form for differential pulse voltammetry.
The potential wave form is composed of regular pulses that are consistently amplified and overlaid onto a staircase-like wave. Here, the current is sampled twice in each Pulse Period (once before the pulse, and at the end of the pulse), and the difference between these two current values is recorded and displayed
Differential pulse anodic stripping voltammetry (DPASV) is important for the determination of for the detection of trace, heavy metal ions in very low concentrations in various sources like drinking water and soil, and in food industry for the determination of food contaminants such as toxic metal, pesticide, fertilizer, etc.
In this experiment a compact and portable potentiostat, PhadkeSTATTM20 which runs on “EC-Prayog” a Windows supporting software was used.
A 3- electrode voltammetry cell, a Glassy carbon working electrode, a silver/silver chloride reference electrode, a Platinum counter electrode, a micropipette. (range from 100ul-1000ul)
The working electrode was cleaned by lightly polishing with alumina and then was rinsed with water. The connection between potentiostat and the cells are: Green= working; White= reference; Red= counter; Black= ground.
Figure 2: From left to right (a) Voltammetry Cell (b) Reference electrode (Ag/AgCl) (c) Counter electrode (Pt Wire) (d) Working electrode (Glassy Carbon).
All solutions should be prepared fresh and all the glassware should be soaked, preferably overnight in 3 M HN03 and rinsed several times with deionised water.
Acetic acid (CH3COOH), ammonia (NH3), Distilled Water, Hg standard stock solution (1000ppm), Hydrochloric acid (HCl) and Cadmium sulphate (3CdSO4.8H2O)
(Cd Standard)
To prepare 1000ppm of Cd standard, weigh out 0.228g of 3CdSO4.8H2O and dissolve in by adding distilled water in a 100ml Standard flask then make it up to the volume and mix well. Then prepare a solution of concentration 10ppm by serial dilution method.
(Ammonium acetate buffer)
11.1 ml of acetic acid was taken in a 100ml volumetric flask, to this 20 ml of distilled water was added. 7.4ml of ammonia was slowly added to the volumetric flask. Ammonia has to be added slowly, because of the generation of heat while addition. After the addition of ammonia, the solution was diluted to 100ml with distilled water. The pH of the buffer is 4.6.
A 20ml solution of Hg2+ is prepared. Take 0.4ml of Hg standard stock solution and put in the 20 ml volumetric flask. Add 0.2 ml of hydrochloric acid to it. Dilute the volumetric flask up to the mark with distilled water. This solution can be reused several times (20) and should be stable for a month.
Used for purging to remove oxygen. Nitrogen is passed through a bottle containing the buffer solution for approx. 30 minutes to prevent evaporation of the sample solution.
Clean the GC electrode with soft tissue, then the electrode is polished with electrode polishing kit; it is explained in detail in this article. The electrode is then rinsed with acetone and afterwards thoroughly with distilled water.
Here TFME is prepared in situ by adding the mercury plating solution directly into the supporting electrolyte at -0.4V for 300 seconds in the DPSV method. TFME can be prepared separately by plating mercury in a different solution then analyte and once the TFME is generated, it must be protected from oxygen to prevent oxidation of the film.

Figure 3: Differential Pulse Stripping Voltammetry

Figure 4: Analysis of voltammogram
The voltammogram overlay for each concentration is showcased below for your reference.
2. The relationship between concentrations and peak currents can be depicted by plotting the peak current (ip) against different concentrations.
Figure 7: The peak current increases linearly with the concentration of the analyte.
Differential pulse Anodic stripping voltammetry is inherently very sensitive voltammetric technique used in water and food analysis for detecting trace metal. Thin film mercury electrode TFME is used for very low levels (ppb) of trace metals analysis. The sensitivity of TFME depends on the electrode’s surface area, lower limit of detection can be achieved by the electrode having larger surface area. Stripping voltammetry can be used for the detection of metals such as Zn, Pb and Cu etc.